Calls for Ukraine
Calls for Europe
Calls for USA
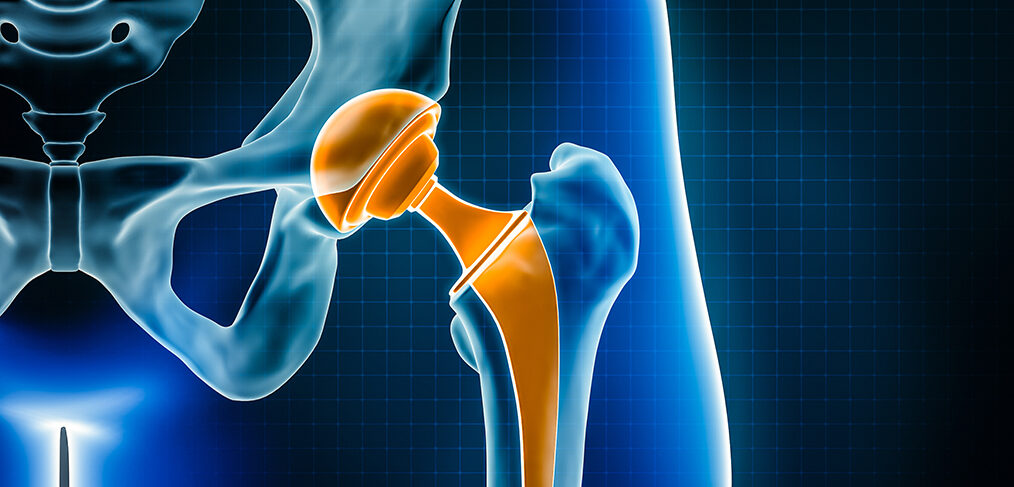
A new study led by experts at the University of Bristol has found that hip implants with a delta ceramic or oxidized zirconia head and a highly cross-linked polyethylene insert or cup have the lowest risk of undergoing repeated surgery (revision) for partial or total joint replacement within 15 years of the first surgery. The results of the study may help hospitals, surgeons, and patients in selecting an implant for hip replacement.
The purpose of the study was to identify the hip implant materials with the lowest risk of revision to assist orthopaedic surgeons and patients in making preoperative collaborative decisions to select hip implants with the lowest risk of revision.
The independent study, published Nov. 7 in the journal PLOS Medicine, was funded by CeramTec and supported by the National Institute for Health Research (NIHR) and the Bristol Center for Biomedical Research.
Researchers analyzed National Joint Replacement Registry (NJR) data from 1,026,481 hip replacement patients over a period of 15 years after their first hip replacement surgery (from 2003 to 2019).
After analyzing the data, the research team found that the risk of revision after hip replacement depends on the type of material used to make the support surface (support surfaces are the moving parts of an artificial hip joint that slide over each other during movement).
Joint replacement surgery is used to treat a variety of musculoskeletal problems, including osteoarthritis and serious injuries. It is a common and very effective surgical procedure. By 2060, the demand for joint replacement will increase by nearly 40 percent from current levels. Joint replacement is long-lasting: more than half of hip and knee replacements last more than 25 years.
Hip implants with a delta ceramic or oxidized zirconia head and a highly cross-linked polyethylene insert or cup were reported to have the lowest risk of revision within 15 years of hip replacement surgery.
These findings were confirmed when the research team looked at the specific reasons for revision hip replacement surgery.
Further research is needed to clarify the relationship of implant materials to the risk of re-hospitalization, reoperations other than revision, mortality, and cost-effectiveness of these materials.
Michael Whitehouse, Professor of Trauma and Orthopaedics at Bristol Medical School and senior clinical lead for the work, explained, “Our study used data from one of the world’s largest registries, including all public and private healthcare providers in the UK. This means that the findings are more universal than those previously available, which were limited to broad categories of implant types or a much smaller study size.”
This study was not a randomized controlled trial, so it was not possible to test all factors that might influence the risk of revision.
National registries for hip replacement surgery often use a broad categorization of implants used, which does not fully identify differences in revision risks associated with different types of implant materials lumped together.
Please rate the work of MedTour
