Calls for Ukraine
Calls for Europe
Calls for USA
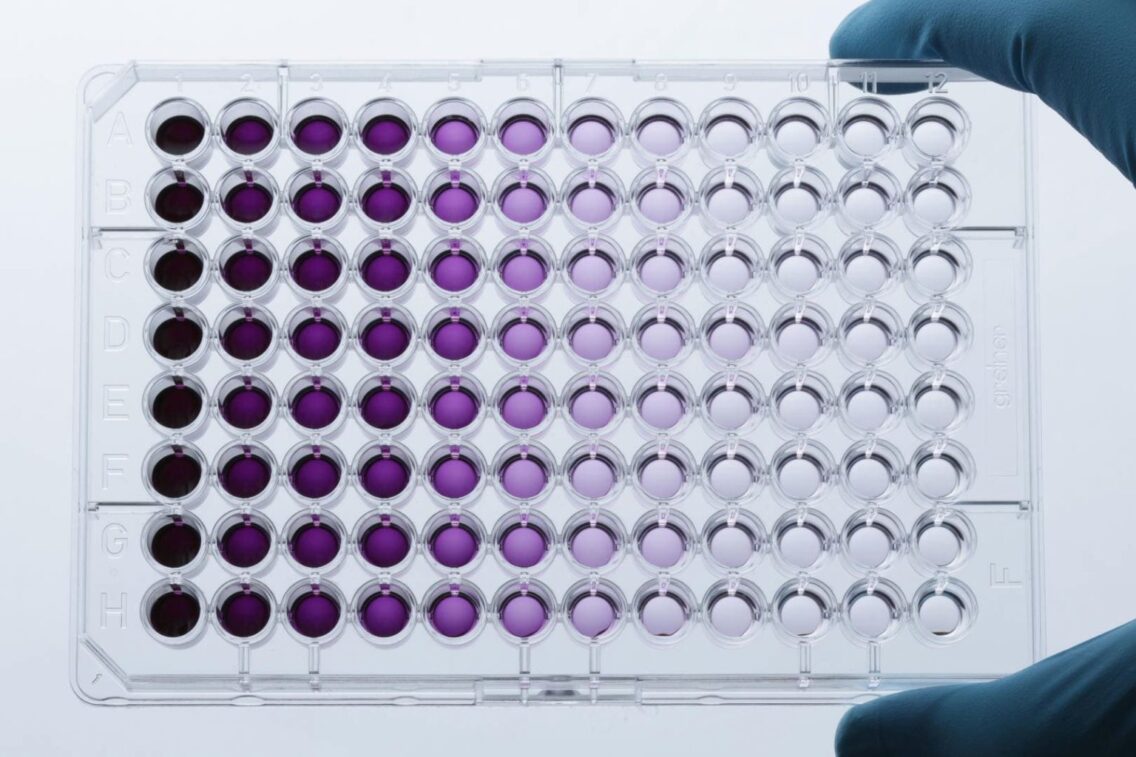
A team of researchers at the University of Texas at El Paso, led by Xiujun (James) Li, Ph.D., professor of biological chemistry, has created a low-cost, portable device that can detect colon and prostate cancer in as quickly as one hour.
This device is much cheaper and faster than existing methods. The device could be particularly useful in developing countries where cancer mortality rates are higher, also because of barriers to meaningful diagnosis.
“The new biochip device is inexpensive, only a few dollars, and is highly sensitive, which will allow accurate diagnosis of the disease for anyone, rich or poor,” says the researcher.
The doctor explained that the most commonly used commercial method for detecting cancer biomarkers, known as ELISA, requires expensive equipment to work correctly and can take twelve hours or more to process a sample. In rural areas of the U.S. or developing countries, he said, this delay is even more pronounced because patient samples have to be transported to major cities with specialized instruments, contributing to higher cancer mortality rates.
The device that Lee’s team created is microfluidic, meaning it can perform multiple functions using very small volumes of fluids. The device uses an innovative “paper in a polymer pond” structure, in which a patient’s blood samples are introduced into tiny wells, after which they fall onto a special kind of paper. The paper captures biomarkers of cancer proteins in the blood samples in just a few minutes. Subsequently, the paper changes color, and the intensity of the color indicates the type of cancer detected and its degree of progression.
So far, the study has focused on prostate and colorectal cancers, but the scientists say the method they have developed could be applicable to a wide variety of cancers.
According to Li, the device can analyze a sample in one hour – compared to 16 hours with a number of traditional methods. According to the study, the device is also about 10 times more sensitive than traditional methods, even without the use of specialized instruments. This means the device can detect cancer biomarkers that are present in smaller amounts, which is typical of early-stage cancer. A less sensitive instrument might not be able to pick up such quantities, Li said.
Before the device can be made available to the public, a prototype will need to be finalized and tested on patients in clinical trials, which could take several years. Before the device can be used by physicians, it must receive final approval from the U.S. Food and Drug Administration and regulatory agencies in other countries.
Dr. Li’s invention greatly improves diagnostics in healthcare settings by reducing detection time and the need for expensive devices. This makes it ideal for use in resource-limited settings, which will improve early diagnosis and lead to better cancer treatment outcomes.
Read more in the journal Lab on a Chip (2024).
Please rate the work of MedTour
