Calls for Ukraine
Calls for Europe
Calls for USA
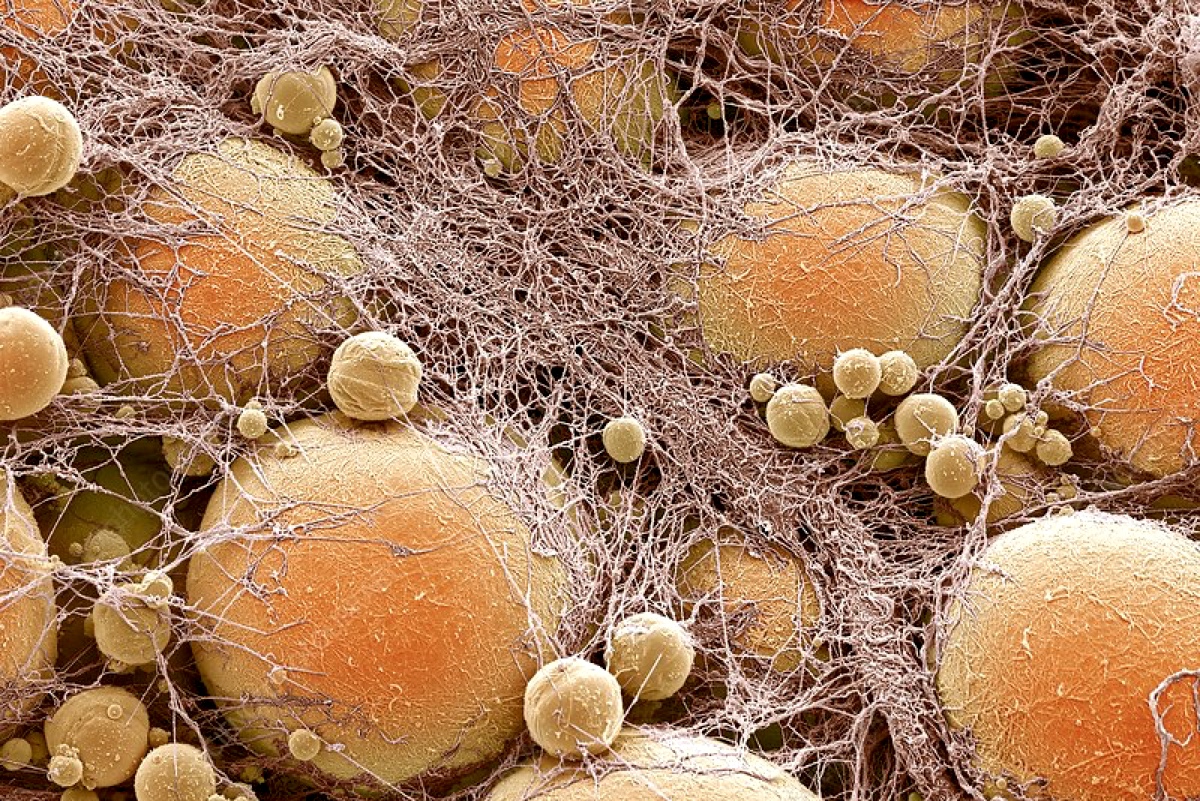
Scientists turned white fat cells that store energy into brown fat cells that burn calories. Once implanted, they overtook cancer cells in the competition for resources, defeating five different types of malignant cells in laboratory experiments.
Liposuction and plastic surgery are rarely mentioned in the same lineup as cancer.
But they are the inspiration for a new approach to cancer treatment that uses engineered fat cells that can deprive tumors of nourishment.
Researchers at the University of California, San Francisco, used CRISPR gene editing technology to turn ordinary white fat cells into “brown” cells that greedily consume calories to generate heat. These cells were then implanted near the tumors, similar to the way plastic surgeons inject fat from one part of the body to enlarge another. The fat cells absorbed all the nutrients, causing most of the tumor cells to starve to death. This approach worked even when the fat cells were implanted into mice far from the sites of their tumors.
“We are already used to removing fat cells with liposuction and injecting them back with plastic surgery,” says Nadav Ahituv, PhD, director of the UCSF Human Genetics Institute and professor in the Department of Bioengineering and Therapeutic Sciences. He is the lead author of a paper published in February in Nature Biotechnology. – These fat cells can be easily and safely manipulated in the lab and placed back into the body, making them an attractive platform for cell therapies, including cancer.”
Ahituv and his colleagues were already aware of studies that showed that exposure to cold can suppress cancer in mice. The scientists concluded that cancer cells starve because cold activates brown fat cells, which use nutrients to produce heat.
But cold treatment is not suitable for cancer patients with compromised health.
So Ahituv and colleagues turned to the idea of using brown fat, believing they could engineer it to burn enough calories even in the absence of cold, depriving tumors of the fuel they need to grow.
The scientists examined genes that are dormant in white fat cells but active in brown fat cells, hoping to find the ones that would turn white fat cells into the hungriest brown fat cells.
A gene called UCP1 came up first.
The researchers then grew brown fat cells with the UCP1 gene along with cancer cells in a Petri dish. The cancer cells were at the bottom and the fat cells above them, in separate compartments that kept the cells apart but forced them to exchange nutrients.
The results were astonishing.
“In our very first experiment, very few cancer cells survived. We thought we were messing something up – we were sure it was a mistake. So we repeated the experiment several times, and all the time we observed the same effect.”
Nadav Ahituv, Principal Investigator
The brown fat cells resisted two different types of breast cancer cells, as well as colon, pancreatic and prostate cancer cells.
But the researchers still didn’t know if the implanted brown fat cells would work in a real-world setting.
To test how they would work in human tissues, the scientists collected samples of breast mastectomies containing both fat and cancer cells.
Because there is a lot of fat in the breast, the scientists decided to take fat from the same patient, modify it, and grow it in the same well with that patient’s own breast cancer cells.
These brown fat cells from the same patient fought off the breast cancer cells in Petri dishes and when implanted together in mouse models.
Knowing that cancerous tumors have their own nutritional preferences, the researchers engineered the fat to consume certain nutrients. For example, some forms of pancreatic cancer “ like” uridine. So they programmed the fat to consume only uridine, and it easily defeated the pancreatic cancer cells.
This suggests that fat can be adapted to the nutritional preferences of any type of cancer.
Fat cells have many advantages when it comes to cell therapy.
They are easy to obtain from patients. They grow well in the lab, and they can be engineered to express different genes and perform different biological functions. They behave well once they return to the body without leaving the implantation site and interact with the immune system.
This fact is supported by decades of progress in plastic surgery.
Fat cells can also be programmed to signal or perform more complex tasks. Their ability to beat cancer, even when they are not near the tumor, could prove invaluable for treating hard-to-treat cancers such as glioblastoma, which affects the brain, as well as many other diseases.
Please rate the work of MedTour
